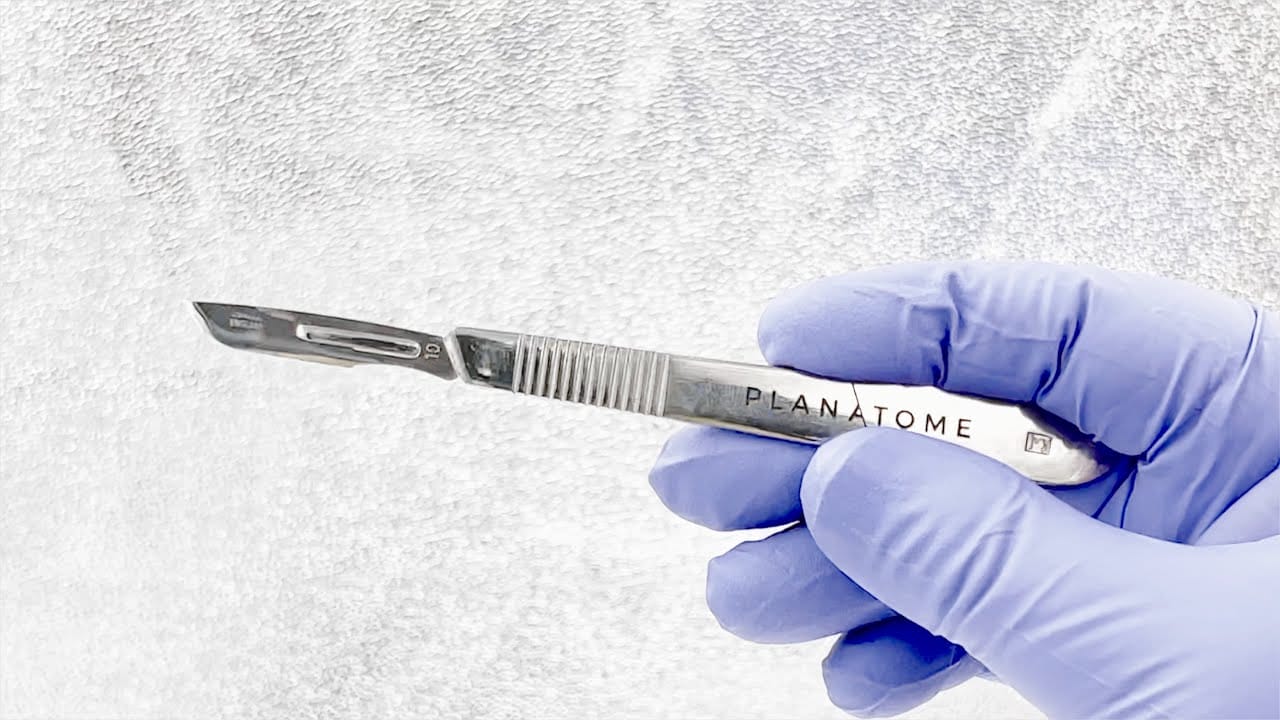
Planatome, a medical device company that applies atomic-level polishing from the semiconductor industry to create advanced surgical blades that lead to better patient outcomes, today announced the plans for the development of a manufacturing plant in the greater Phoenix area and to more than double the size of its workforce over the next five years.
LEARN MORE: ACA attracts billions in investment to Arizona
“We are grateful for the investment and support we have received from the Arizona Commerce Authority and the State of Arizona,” said Tim Tobin, Planatome’s chief executive officer. “This investment demonstrates that Arizona supports businesses and innovation. Planatome is growing rapidly, and we are expanding the reach of our surgical blades into new practice fields. We are committed to growing our business operations here and a long productive relationship with the state.”
The Arizona Commerce Authority will also support Planatome through the Arizona Manufacturing Extension Partnership (AZ MEP), which helps Arizona manufacturers achieve their business goals by connecting them to resources, including workforce solutions, supply chain optimization, and more.
“Planatome’s manufacturing facility will highlight Arizona’s impressive reputation for emerging technologies and innovation,” said Sandra Watson, president and CEO of the Arizona Commerce Authority. “With the planned facility, Planatome will join a growing list of manufacturers making products here in Arizona, adding to our vibrant medical and biotech industry.”
Planatome’s patented surface-modification technology is based on chemical mechanical planarization (CMP), a process used to planarize silicon wafers in advanced chip manufacturing. The process dramatically improves the surface smoothness of surgical blades. Surgeons who use Planatome blades see less soft-tissue damage at the incision, less closing resistance, cleaner margins and fewer complications. Their patients experience less inflammation and pain along with a quicker recovery and smaller, less noticeable scarring. The blades are approved for use in the United States and South Korea and have recently been approved for use by several hospitals and surgical centers.
“Planatome blades are a game changer,” said Dr. Anthony Admire of Admire Plastic Surgery in Scottsdale, Arizona. “The blades are a major innovation in surgery. Planatome reduces scarring at the time of incision with a smooth cut and clean margins with no rough edges. This ability to control scarring at the start of the procedure rather than postoperatively is a great innovation in my practice. My patients have absolutely benefitted from my use of this blade. I’m looking forward to future innovations and think it’s great that the company will expand operations in Arizona.”
The company is currently researching how its technology can be used for other medical device applications, including surgical scissors, laparoscopic tools and robot end effectors. Planatome’s technology also can be adapted and applied to several other industries with a wide range of products that could benefit from having an atomically smooth surface.
Planatome blades are now in production and may be purchased from Planatome directly. For more information, visit https://planatome.com.
To learn more about Planatome, follow the company on LinkedIn: Planatome.